Research Highlights
NCMIR and Salk Researchers “animate” synaptic transmission
July 2005
In a collaboration between computational neuroscientists and neuroanatomists, researchers have created a 3-D, nano-scale “anatomically correct” neuronal synapse that provides new insights into the way synapses work.
Efforts to model activities of the nervous system have generally required drastically simplifying the complex geometry of the brain and its microscopic components. A look under the electron microscope, however, reveals that the brain is a very crowded place, made up of many sub-micron parts of nerve cells with very narrow spaces between them. New techniques in 3D electron microscopy also show that synapses and their associated components come in many shapes and sizes, complete with peaks and valleys and other interesting formations. It is within these complicated geometrical microdomains that synaptic transmission takes place, at specialized contact sites between nerve cells. Why does one type of synapse look so different than another and what effect does the spatial configuration of surrounding components have on cell-to-cell signaling?
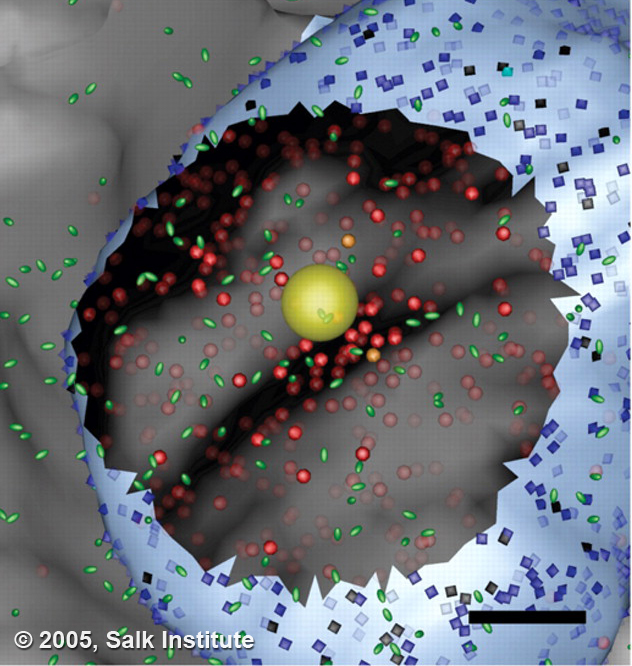
It would be great if neuroscientists could peer into the sub-micron world revealed by electron microscopy and watch as neurotransmitters released by one cell diffuse through the space and activate their post-synaptic targets. Unfortunately, the technology to achieve this doesn't exist. In the electron microscope, which is currently the only instrument capable of resolving sub-micron structures, all action is stopped. Each image represents a snapshot frozen in time of a very dynamic and crowded space.
In the study by Coggan and colleagues, published in the July 15 issue of Science, researchers were able to “animate” synaptic transmission in a spatially realistic microdomain by combining 3D electron microscopic reconstructions with advanced computational simulation and visualization tools.
Using an advanced imaging technique called electron tomography, they created a detailed computer model of the synaptic microdomain of a neuron in the ciliary ganglion, a region of the nervous system that controls the lens of the eye. A synapse is formed between a very large claw-like axon and the surface of the ciliary ganglion cell, which consists of many small, flattened fingers lying against the surface of the cell. The axon releases a transmitter called acetylcholine, which binds to receptors contained in synaptic zones on the post-synaptic cell.
Coggans showed that the diffusion of neurotransmitter is highly influenced by the topology of synaptic components. They also provided intriguing, and possibly controversial, evidence that neurotransmitters act not only at morphologically identified synaptic sites, but also along non-synaptic regions of the post-synaptic cell. This work shows how new combinations of experiment and computation can be used to shed light on nano-scale cell and tissue-level behaviors that cannot be studied through direct experimentation alone. In the future, these biologically realistic models will have broad and deep impact on treatment of diseases using precisely designed drug and gene therapies.
Coggan JS, Bartol TM, Esquenazi E, Stiles JR, Lamont S, Martone ME, Berg DK, Ellisman MH, Sejnowski TJ (2005) Evidence for ectopic neurotransmission at a neuronal synapse. Science Jul 15;309(5733):446-51.