Research Highlights
Adenovirus Protein may be a Key to Strategic New Cancer Therapies
January 2013 La Jolla
A team of scientists led by Clodagh O’Shea’s group at The Salk Institute for Biological Studies in collaboration with NCMIR scientists (Mark Ellisman, Tom Deerinck, Andrew Noske) and Dr. Roger Tsien and his laboratory suggest that cold viruses could serve as a valuable tool in the fight against cancer. Adenovirus, a type of cold virus, has developed proteins that allow it to hijack a cell's molecular machinery particularly those involved in growth, replication and cancer suppression.
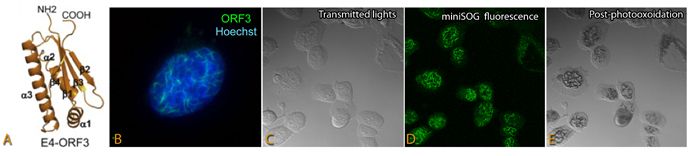
E4-ORF3 is a cancer-causing protein encoded by adenovirus, which prevents the p53 tumor suppressor protein from binding to its target genes. Known as the "guardian of the genome," p53 normally suppresses tumors by causing cells with DNA damage to self-destruct. The p53 tumor suppressor pathway is inactivated in almost every human cancer, allowing cancer cells to escape normal growth controls. When E4-ORF3 inactivates p53, adenovirus replication in infected human cells goes unchecked. E4-ORF3 helps to deactivate genes that help the cell defend itself against adenoviruses. E4-ORF3 self-assembles inside the cell’s nucleus into a disordered web-like structure that captures and inactivates different tumor suppressor protein complexes. It polymerizes into all sorts of different shapes and sizes that can capture and deactivate the many defenses of a host cell. The findings suggest a new avenue for developing cancer therapies by mimicking the strategies employed by these viruses.
In collaboration with scientists from the NCMIR, O'Shea's team used advance imaging technologies, such as miniSOG genetic labeling, high resolution electron tomography and serial block face scanning electron microscopy, to reveal the ultrastructure of the remarkable polymer that E4-ORF3 assembles in the nucleus. The polymer is barely detectable using conventional electron microscopy. Initially, E4-ORF3 forms a dimer, and ignores its cellular targets. The researchers theorized that when E4-ORF3 assembles into a polymer, however, it binds to tumor suppressor targets far more aggressively. To test this theory, they genetically fused E4-ORF3 polymer mutants to lamin, a cellular protein that assembles intermediate filaments that provide stability and strength to cells. They showed that the lamin-E4-ORF3 fusion protein assembled into cylinder-like superstructures in the nucleus that bind and disrupt PML, a protein complex that suppresses tumors.
Understanding how viruses overcome healthy cells may also help scientists engineer tumor-busting viruses, which offer a new and potentially self-perpetuating cancer therapy. Such modified viruses would destroy only cancer cells, because they could only replicate in cells in which the p53 tumor suppressor has been deactivated. When a cancer cell is destroyed it would release additional copies of the engineered viruses, which would seek out and kill remaining cancer cells that have spread throughout the body. Engineering these viruses requires disabling the ability of the E4-ORF3 protein to inactivate p53 in healthy cells. The virus could otherwise destroy healthy cells as well as cancer cells. E4-ORF3 plays such an important role in viral replication, it can not be completely removed from the virus's replication machinery. A different strategy of to re-engineering viruses in which E4-ORF3's abilities have been tailored to fight cancer rather than causing colds will require understanding the protein's precise structure, functions and interactions.
The work was supported by the National Institutes of Health, American Cancer Society, Sontag Foundation, the Arnold and Mabel Beckman Foundation, and Anna Fuller Foundation.
Adapted from: http://www.sciencedaily.com/releases/2012/10/121016162830.htm
Citation: Horng D. Ou, Witek Kwiatkowski, Thomas J. Deerinck, Andrew Noske, Katie Y. Blain, Hannah S. Land, Conrado Soria, Colin J. Powers, Andrew P. May, Xiaokun Shu, Roger Y. Tsien, James A.J. Fitzpatrick, Jeff A. Long, Mark H. Ellisman, Senyon Choe, Clodagh C. O'Shea. A Structural Basis for the Assembly and Functions of a Viral Polymer that Inactivates Multiple Tumor Suppressors. Cell, 2012; 151: 304-319.