Research Highlights
New Insight Toward Understanding the Molecular Basis for Long-term Memory
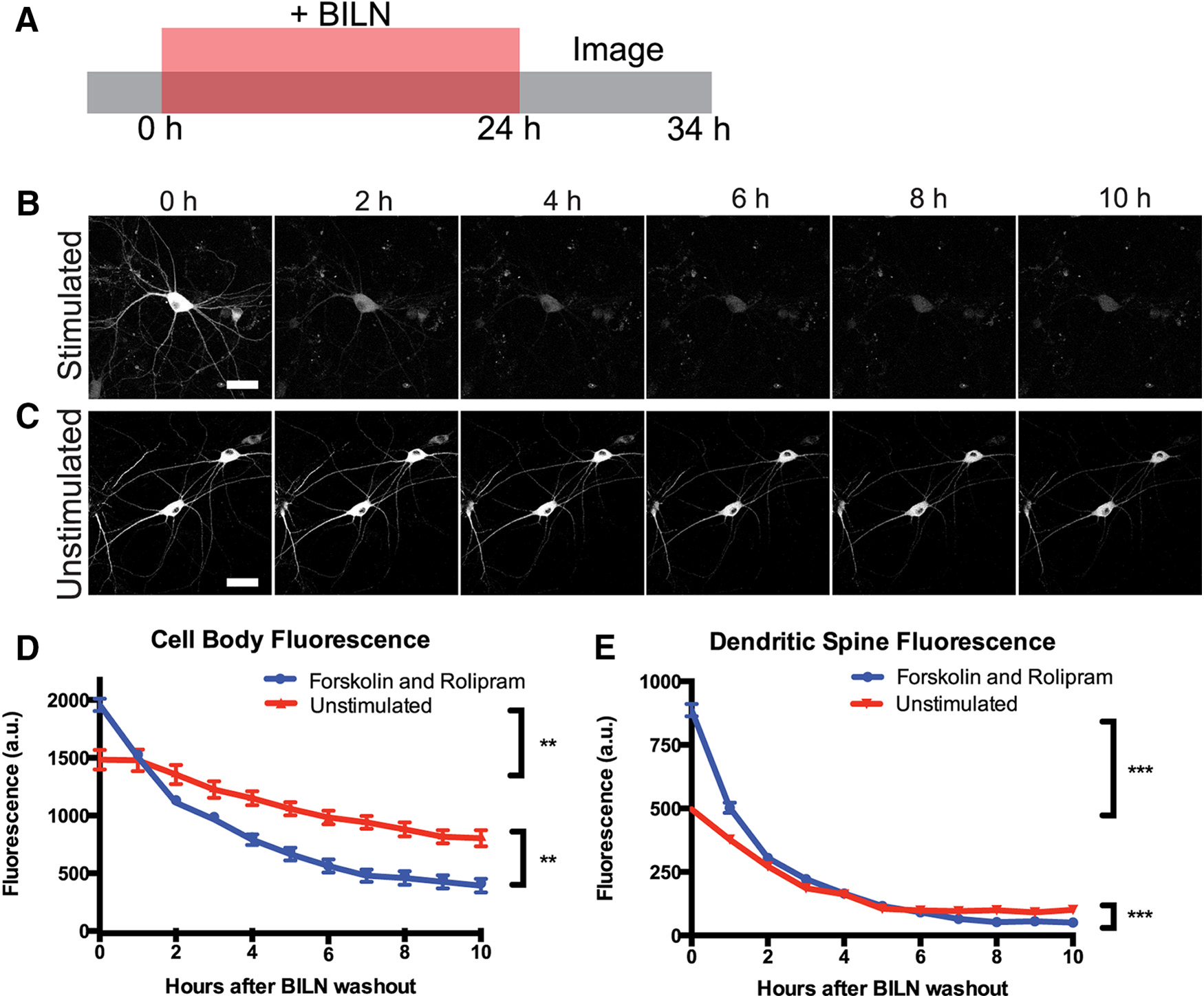
TS:YSOG3-PKMζ degrades rapidly in neuronal cell bodies and dendritic spines after forskolin and rolipram cLTP. A, Schematic indicating how neurons transfected with TS:YSOG3-PKMζ were treated with BILN for 24 h, then imaged once per hour for 10 h after BILN washout.B, Stimulated neuron YFP fluorescence decreased quickly over time, indicating that PKMζ is rapidly degraded. C, Unstimulated neuron YFP fluorescence decreased slowly in the 10 h following BILN washout, indicating that basal PKMζ remains more stable over time. D, YFP quantification in neuron cell bodies. E, YFPquantification in dendritic spines.
20-05-2015 La Jolla
Human memory can persist for a lifetime, yet the majority of synaptic proteins in the brain turn over at a rate of hours to days. Synthesizing, localizing, and stabilizing new protein copies at synapses are crucial factors in maintaining the synaptic changes required for storing long-term memories. For memories to remain stable, the changes made to encode the memory must also perpetuate themselves as the molecules undergo repeated cycles of generational turnover. But the mechanisms by which long-lasting memories are retained in this dynamic synaptic environment remain unknown.
New protein synthesis is crucial for long-term potentiation (LTP) and long-term memory (LTM). Recently, PKMζ has been proposed as the primary molecule for maintaining encoded memories over time because its presence correlates with late LTP and its inhibition via the pseudosubstrate peptide ZIP disrupts LTP in vitro and long-term memory storage in vivo.
But recent studies have questioned the role of PKMζ in LTP and memory by demonstrating that ZIP is not specific for PKMζ: ZIP may not effectively inhibit PKMζ in vivo, or it may inhibit other synaptic molecules in addition to PKMζ that may be the true mediators of LTP and LTM. Such studies indicate that mice lacking PKMζ have normal LTP and memory. An alternative target for ZIP is PKCλ, a PKC isoform that is similar to PKMζ and is also inhibited by ZIP. Complicating this issue is that PKMζ and PKCλ are known to have different functions during neuronal development but have similar substrates and may act redundantly or compensate for each other during LTP maintenance.
To gain insight into whether these two proteins, PKMζ and PKCλ, could independently sustain long-lasting changes in synaptic strength, a research team from UC San Diego and the Howard Hughes Medical Institute investigated PKMζ and PKCλ stability in rat neurons to better understand their roles during information encoding and storage. The team developed a modified TimeSTAMP (TS) reporter to track PKMζ and PKCλ synthesis, degradation, turnover, and stability in response to chemical LTP stimulation.
TS reporters were designed originally to characterize the production and lifetime of a specific protein of interest. New versions incorporate the split yellow fluorescent protein (YFP) Venus and the mini singlet oxygen generator tag (miniSOG, a genetically encoded tag used in correlated light and electron microscopy) to create fluorescence that can be imaged in high temporal and spatial resolution by electron microscopy.
The team modified the existing Venus/miniSOG reporter to produce a new TS reporter for correlated light and electron microscopy that can identify both new and old copies of a protein fused to TS via its N terminus. (The older version of the reporter was designed to fuse to the C terminus.) They then used this reporter to visualize PKMζ and PKCλ synthesis and degradation in cultured rat neurons following chemical LTP stimulation to characterize the stability of labeled proteins over time. New copies of tagged protein were made fluorescent in a drug-dependent manner, while old copies remained unlabeled and invisible. Old protein copies could be monitored by removal of the drug; reduction in fluorescence over time was due to protein degradation.
More PKMζ protein was produced in stimulated neurons as expected. However, surprisingly, this population of PKMζ had an elevated rate of degradation. In contrast, PKMζ in unstimulated neurons was produced at a lower level, but most of this protein remained relatively stable. The observation that PKMζ is both rapidly synthesized and degraded following stimulation suggests that a stable amount of PKMζ would be difficult to maintain at synapses and perpetuate memory storage over long time scales.
To characterize new PKCλ synthesis, the team fused the reporter to PKCλ and visualized PKCλ production and degradation. In contrast to PKMζ , PKCλ strongly localized to synapses both with and without stimulation. Unlike PKMζ, PKCλ turnover was unaffected by stimulation, and new copies of PKCλ appeared to remain more stable at synapses over time. This work demonstrates that only new PKCλ copies remain stable at these synapses during longer time scales of more than a day and suggests that PKCλ may have a different synaptic role than PKMζ.
In conclusion, this work addresses the stability of synaptic memory-related proteins PKMζ and PKCλ and demonstrates that TS reporters are useful tools to visualize and track specific populations of a given protein over time. These reporters could be used in the intact brain to identify synapses modified after a learning experience that maintain the memory of what was learned. Experiments using confocal brain tissue imaging and live animal brain imaging to visualize TS-tagged proteins synthesized in response to stimulation or a learning paradigm could provide further insight into long-lasting synapse modifications.
Funding Source: This work was supported by National Institutes of Health Grant NS027177 to R.Y.T. and Grants R01 GM086197 and P41 GM103412 to M.H.E., S.F.P. was supported by the University of California, San Diego, Graduate Training Program in Cellular and Molecular Pharmacology (T32 GM007752) and the University of California, San Diego, Graduate Training Program in Neuroplasticity of Aging (T32 AG000216).
Relevant Publication: Palida, Sakina F., Butko, Margaret T., Ngo, John T., Mackey, Mason R., Gross, Larry A., Ellisman, Mark H., and Tsien, Roger Y. (2015). PKMζ, But Not PKCλ, Is Rapidly Synthesized and Degraded at the Neuronal Synapse, The Journal of Neuroscience, 35(20):7736-7749.